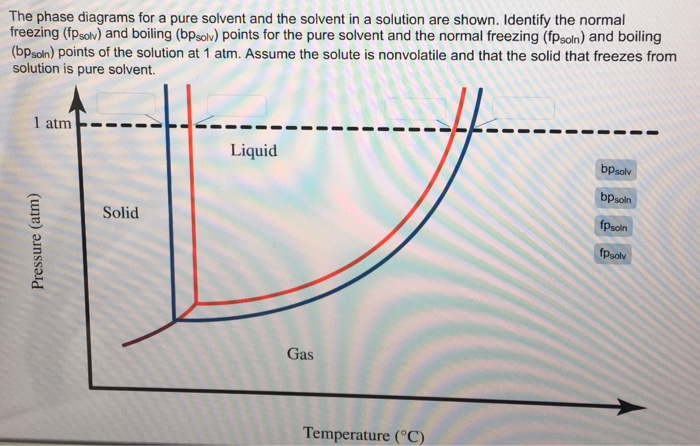
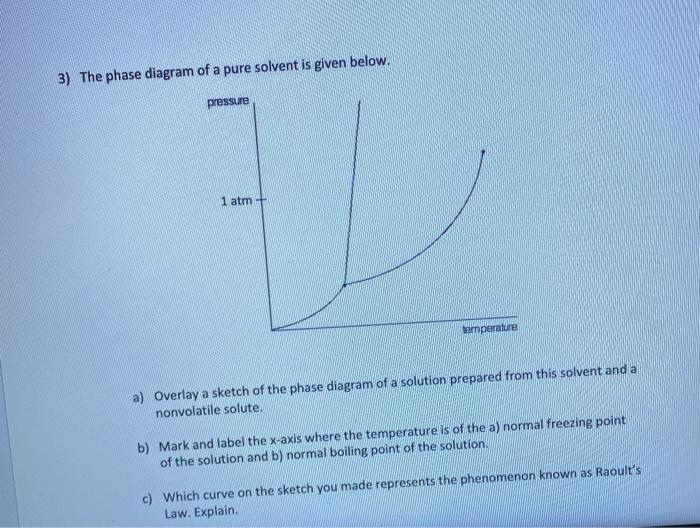
label the phase diagram of pure solvent and a solution
If we place a compound which contains n pi and pi orbitals into a polar solvent the solvent will stabilizes these. By measuring the angles and intensities of these diffracted beams a crystallographer can produce a three-dimensional picture of the density of electrons within the.
Chemistry 104 Molecular Weight By Freezing Point Depression
Figure 21 top shows the methanolchloroformwater ternary phase diagram with the tie-lines in the biphasic domain.
. Calculate the vapor pressure of an aqueous solution containing 302 ethylene glycol by mass a concentration commonly used in climates that do not get extremely cold in winter. The HPLC technique makes use of a stationary phase confined to either a glass or a plastic tube and a mobile phase comprising aqueousorganic solvents which flow through the solid adsorbent. When the sample to be analyzed is layered on top of the column it flows through and distributes between both the mobile and the stationary phases.
Urea is a nitrogenous compound containing a carbonyl group attached to two amine groups with osmotic diuretic activity. The stationary phase and the mobile phase. Recall from Chapter 1 that solutions are defined as homogeneous mixtures that are mixed so thoroughly that neither component can be observed independently of the other.
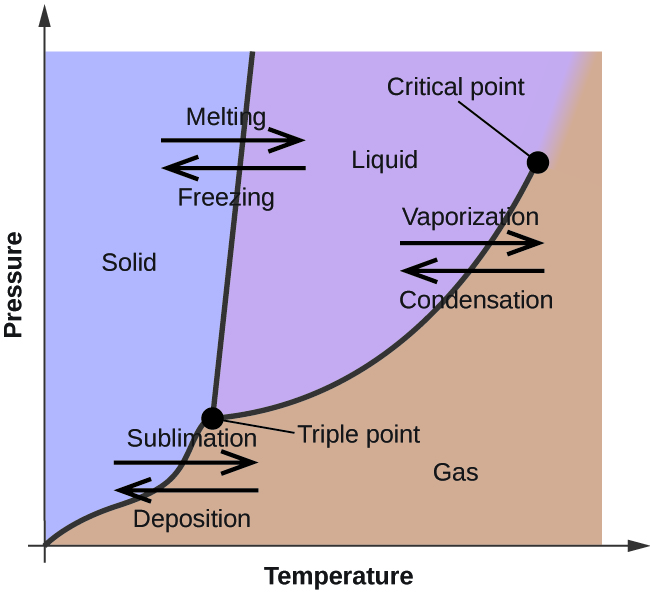
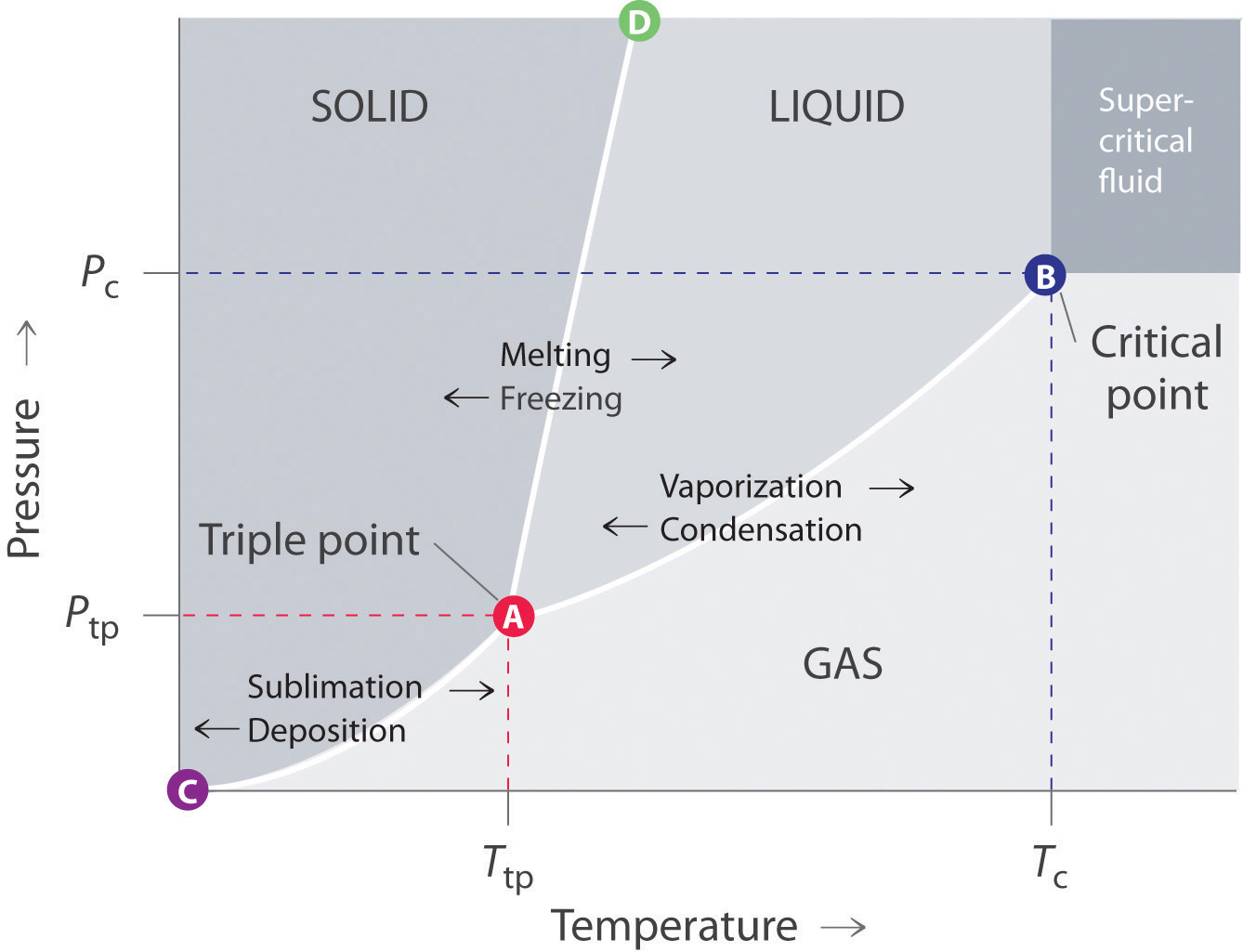
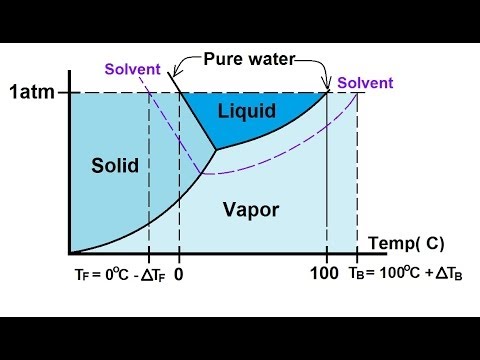
Also called ethyl alcohol grain alcohol drinking alcohol or simply alcohol is an organic compoundIt is a simple alcohol with the chemical formula C 2 H 6 OIts formula can be also written as CH 3 CH 2 OH or C 2 H 5 OH an ethyl group linked to a hydroxyl groupEthanol is a volatile flammable colorless liquid with a characteristic wine-like odor and. Write your answer in column 6. These meet at a single point called the triple point where all three phases can coexistThe triple point is at a temperature of 27316 K 001 C and a pressure of 611657 pascals 000604 atm.
Solutions are all around us. The mobile phase is the. Representative catalysts used industrially are sodium hydroxide potassium hydroxide potassium carbonate and anion-exchange resins.
Ferrum and atomic number 26. N-Butyl chloride was formed during the chlorination of leachate obtained from a simulated landfill used to study the codisposal of metal plating. Solutions are all around us.
Maintaining a Balance 1. Vapor-phase dimethyl carbonate is degraded in the atmosphere by reaction with photochemically-produced hydroxyl radicalsSRC. The paper or more precisely the water that is adsorbed to the paper molecules is the stationary phase and the alcohol and water solution is the solvent mobile phase.
The rate constant for the vapor-phase reaction of cyclopentanol with photochemically produced hydroxyl radicals has been measured as 107X10-12 cu cmmolecule-sec at 25 C1. This lets us find the most appropriate writer for any type of assignment. Ternary phase diagrams are used to represent all possible mixtures of three solvents 1.
Once the pigments have stopped moving remove the paper from the beaker and mark where the solvent stopped. 6 to 30 characters long. Five particular compositions are shown in the.
In vivo urea is formed in the liver via the urea cycle from ammonia and is the final end product of protein metabolism. The reaction usually is carried out in the liquid phase. Chromatography consists of two phases.
ASCII characters only characters found on a standard US keyboard. Must contain at least 4 different symbols. The equilibrium constant is 28 Lmol at 20-25 C.
On a pressuretemperature phase diagram see figure there are curves separating solid from vapor vapor from liquid and liquid from solid. The half-life for this reaction in air is estimated to be 246 daysSRC calculated from its rate constant of 044X10-12 cu cmmolecule-sec at 25 CSRC that was derived using a structure estimation method3. The dowel or rod will suspend the paper in the solvent so it travels up the filter paper and separates the pigments in the ink.
Administration of urea elevates blood plasma osmolality resulting in enhanced flow of water from tissues including the brain cerebrospinal. It may be released to the environment in emissions or wastewater related to its manufacture and these uses. Air for example is a solution.
In a solution the particles are too small that they cannot be seen by the unaided eye. The ink will be spotted onto strips of chromatography paper and put in a beaker containing a solution of alcohol and water. Iron ˈ aɪ ər n is a chemical element with symbol Fe from Latin.
It is a metal that belongs to the first transition series and group 8 of the periodic tableIt is by mass the most common element on Earth right in front of oxygen 321 and 301 respectively forming much of Earths outer and inner coreIt is the fourth most common. The reaction is reversible but formation of the cyanohydrin is quite favorable. Most organisms are active in a limited temperature range IDENTIFY THE ROLE OF ENZYMES IN METABOLISM DESCRIBE THEIR CHEMICAL COMPOSITION AND USE A SIMPLE MODEL TO DESCRIBE THEIR SPECIFICITY ON SUBSTRATES.
IDM Members meetings for 2022 will be held from 12h45 to 14h30A zoom link or venue to be sent out before the time. It is the lowest pressure at which liquid water can. Chromatography is a group of techniques including paper chromatography that are used to separate the various components in a complex mixture or solutionIn each chromatography apparatus there is generally a mobile phase which is a fluid that runs along the stationary phase and a stationary phase that stays stationary while the mobile phase moves through.
H 2 O molecule is a. If you live near a lake a river or an ocean that body of water is not pure H 2 O but most probably a solution. In Activity 2 you found out that a solution is formed when a solute dissolves in a solvent to form a single phase that appears uniform throughout.
X-ray crystallography is the experimental science determining the atomic and molecular structure of a crystal in which the crystalline structure causes a beam of incident X-rays to diffract into many specific directions. Vapor-phase 1-chloropropane will be degraded in the atmosphere by reaction with photochemically-produced hydroxyl radicals. A solution is clear.
Air for example is a solution. Identity of solute percentage by mass and vapor pressure of pure solvent. The polarity of solvent will have an influence on the IR spectra of organic compounds due to the interactions between solvent and compounds which is called solvent effects.
Recall from Chapter 1 that solutions are defined as homogeneous mixtures that are mixed so thoroughly that neither component can be observed independently of the other. 111-Trichloroethane CCl3CH3 or C2H3Cl3 CID 6278 - structure chemical names physical and chemical properties classification patents literature biological. At 100C the vapor pressure of pure water is 760 mmHg.
If released to air a vapor pressure of 344 mm Hg at 25 C indicates 1-chloropropane will exist solely as a vapor in the atmosphere. N-Butyl chloride is used as a solvent an alkylating agent and an antihelmintic medicine2. The solvent will move by capillary action.
The half-life for this reaction in air is estimated to be 15 days. If you live near a lake a river or an ocean that body of water is not pure H 2 O but most probably a solution. Which of the samples are solutions.
They are described in Chapter 3Here we shall indicate how they should be used to minimize the solvent consumption. Our global writing staff includes experienced ENL ESL academic writers in a variety of disciplines. This corresponds to an atmospheric half-life of about 15 days at an atmospheric concentration of 5X105 hydroxyl radicals per cu cm1SRC.
10 4 Phase Diagrams Chemistry Libretexts
Phase Diagrams
8 2 Phase Diagrams Of Pure Substances Chemistry Libretexts
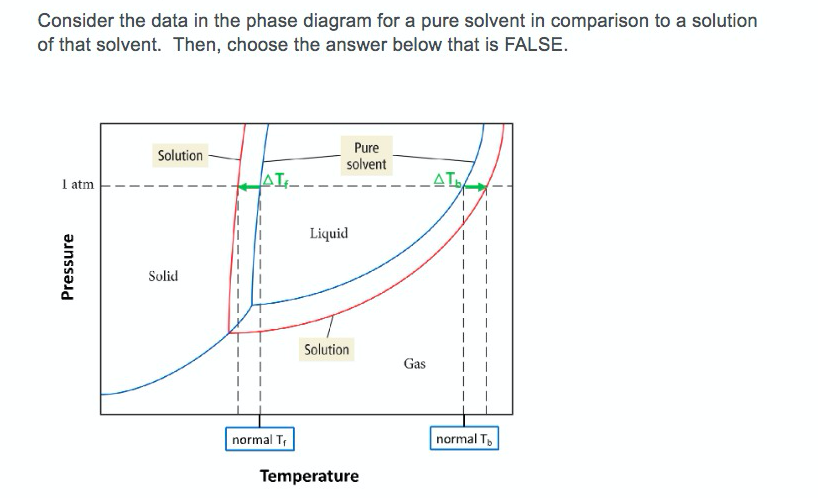
Solved Consider The Data In The Phase Diagram For A Pure Chegg Com
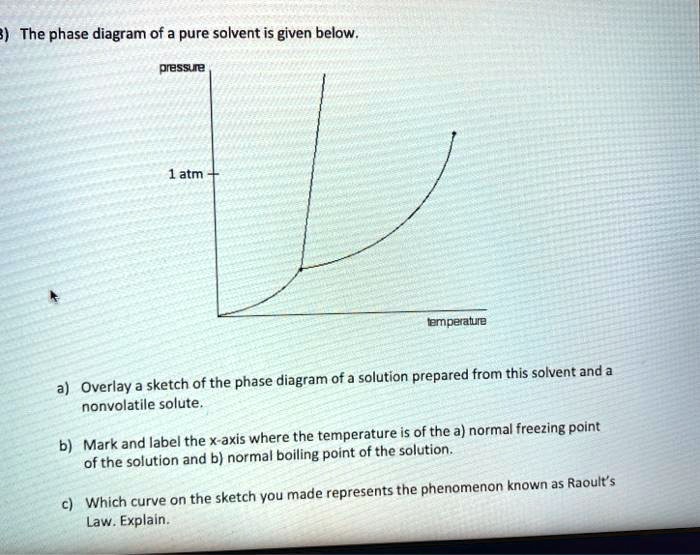
Solved The Phase Diagram Of A Pure Solvent Is Given Below Pressue Atm Lemperaluie Sketch Of The Phase Diagram Of A Solution Prepared From This Solvent And Overlay Nonvolatile Solute Where

Equilibrium Phase Diagram An Overview Sciencedirect Topics

Oneclass Label The Phase Diagram Of A Pure Solvent And A Solution
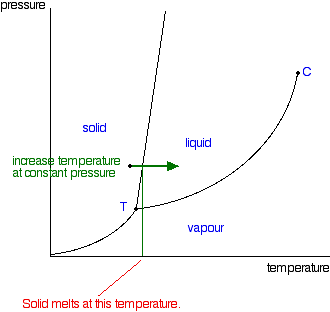
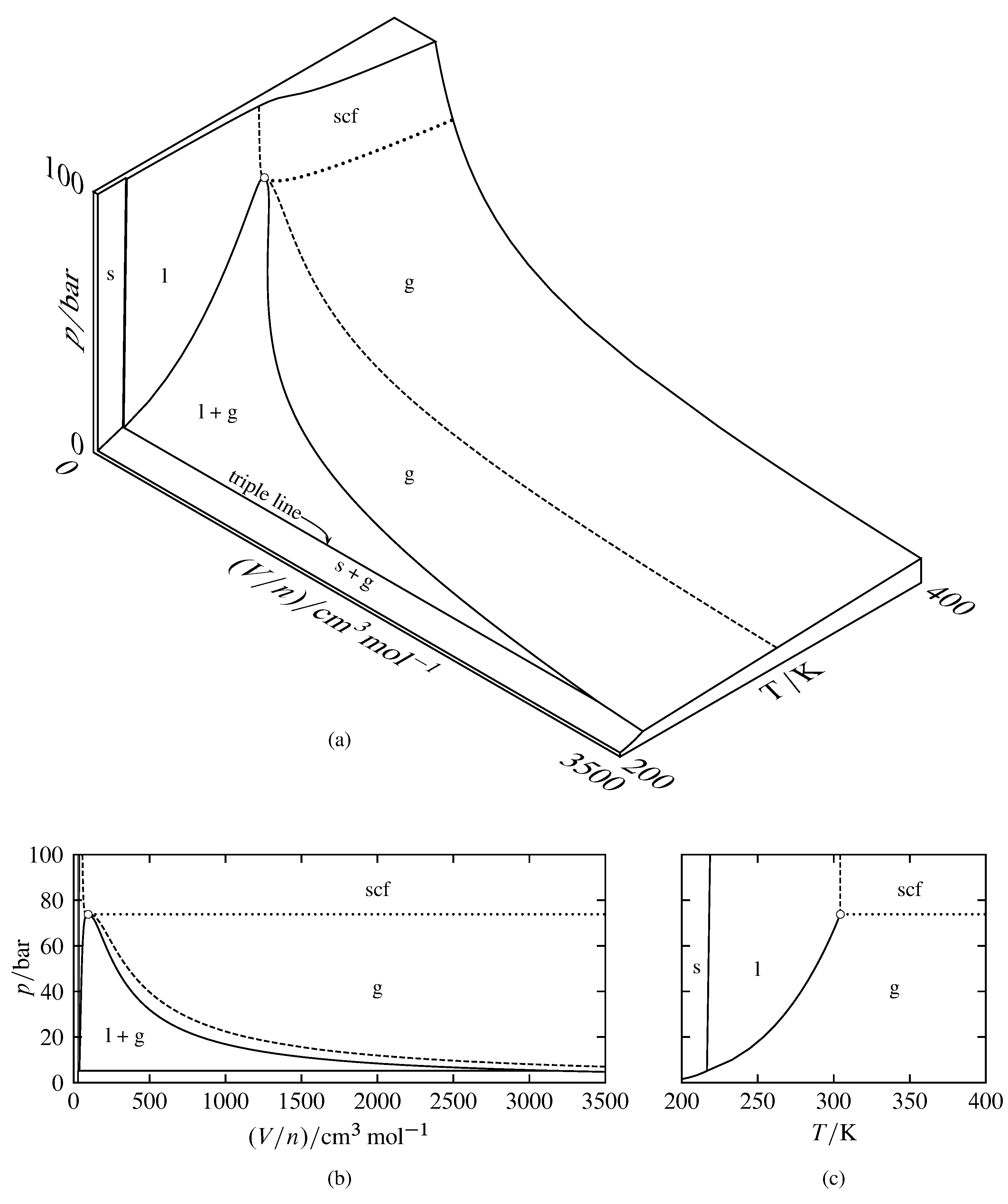
Phase Diagrams Of Pure Substances

Ternary Phase Diagram An Overview Sciencedirect Topics

Media Portfolio

Phase Diagrams Chemistry Atoms First

Oneclass Label The Phase Diagram Of A Pure Solvent And A Solution

Liquid Solid Phase Diagram An Overview Sciencedirect Topics
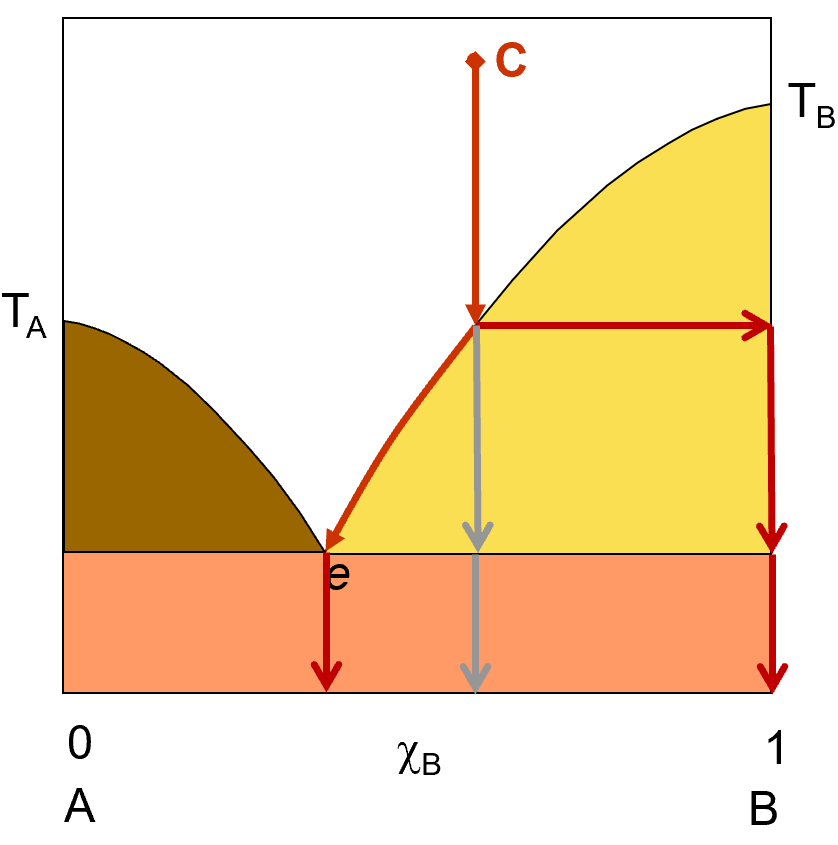
Liquid And Solid Solution Phase Changes First Year General Chemistry
Solved Each Page Part 1 1 Calculate The Vapor Pressure Chegg Com
Chemistry Solutions 40 Of 53 Colligative Properties Phase Diagram Youtube

Phase Diagrams Chemistry Atoms First